Background: Sickle cell disease (SCD) is a genetic red blood cell disorder associated with increased morbidity and mortality. Newborn screening for SCD was introduced in January 2007 in the Netherlands to enable early diagnosis and initiation of preventive measures. The objective of this study was to assess the effect of newborn screening on the morbidity and mortality of children living with SCD in the Netherlands, 16 years after its implementation.
Methods: In this national cohort study all children with SCD diagnosed by newborn screening were included. Patients were followed between 1 January 2007 and 31 March 2023 until end of follow-up, death or successful stem cell transplantation. Following informed consent, data were collected from medical files on SCD genotype, treatment, mortality and development of SCD-related complications. The incidence rate of SCD-complications was presented as lifetime-event per 100 person-years for the total duration of follow-up and for the age categories: 0-4, 5-11, and 12-16 years.
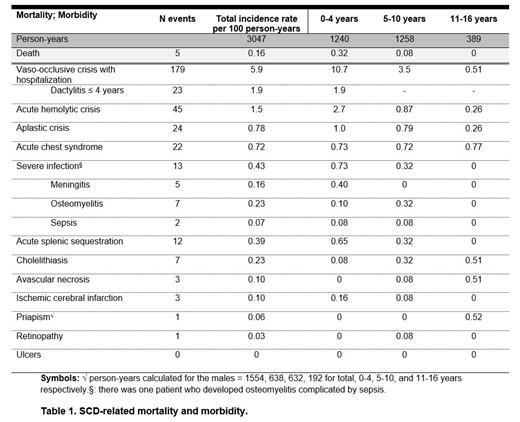
Results: In total, 391 out of 540 eligible patients (72%) with SCD were included in all hospitals with patients with SCD under treatment, accounting for 3047 person-years. The mean age at the end of follow-up was 7.8 years (SD±4.5). The majority of patients had SCD genotype HbSS (58%), followed by HbSC (28%). As for the mortality, five patients died during follow-up, primarily at or below the age of 2 years (4/5, 80%). In three patients, the cause of death was related to SCD, including infection (n=2; aged 1.2 and 2.0 years) and acute splenic sequestration (n=1; 2.0 years). The SCD-related complications are presented in Table 1. The most common SCD complication in this cohort was vaso-occlusive crisis (including dactylitis) with hospitalization. SCD-related complications that mainly developed within the first 4 years of life, were vaso-occlusive crisis, acute hemolytic crisis, aplastic crisis, severe infections, splenic sequestration and ischemic cerebral infarction. Acute chest syndrome occurred equally in all age categories. Other SCD-related complications developed to a lesser extent in our cohort, and at a later age in adolescence or adulthood.
Conclusion: In this cohort of neonatally screened children with SCD, a substantial number of SCD-complications occurred in the first 4 years of life. This study underlines the importance of parental education and 24/7 availably, high level acute specialized clinical care for these children from birth on. Further analysis to identify risk factors at young age for a severe phenotype will be conducted, to enable more targeted interventions in a timely manner. Hopefully, this will lead to reduction of disease burden, improving the quality of life in children living with SCD in the Netherlands.
Disclosures
Cnossen:Roche, Bayer and Novartis: Membership on an entity's Board of Directors or advisory committees; NWO, NWA, ZonMW, the Dutch Innovatiefonds Zorgverzekeraars, Stichting Haemophilia, Baxter/Baxalta/Shire/Takeda, Pfizer, Bayer Schering Pharma, CSL Behring, Sobi Biogen, Novo Nordisk, Novartis, Roche, and Nordic Pharma: Research Funding. Fijnvandraat:Sanofi: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy; Novo Nordisk: Consultancy, Research Funding; CSL Behring: Research Funding; Sobi: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal